Identify Diagnostics Drug Test Cup Training
Certified Training
Identify Diagnostics training program is intended to provide a means for a drug test administrator to develop an understanding of the Identify Diagnostics Cup product, successfully complete the certification, and acquire an understanding of the role drug testing devices fill in a drug testing program. Please read through all of the information within this training program. After completing the training program, you may test your knowledge with our online certification test. Once you have successfully passed the test with 100% accuracy, you will receive a personalized certificate of training in which you can print for your records.
Overview
The Identify Diagnostics Cup is a preliminary screening test for the presence of drugs. These cups or dips provide qualitative, immunoassay urine-based, rapid test that incorporates collection of a urine specimen and the testing of multiple drugs simultaneously. For a quantitative result or to confirm a presumptive positive screen, a second, alternative chemical method should be performed on the same urine sample. Liquid Chromatography / Tandem Mass Spectrometry (LC-MS/MS) or Gas Chromatography / Mass Spectrometry (GC/MS) are the preferred confirmation methods.
Drug Concentrations
The “Cutoff Level” is the concentration level established as a break-point or threshold for labeling a result positive or negative (e.g. 50ng/mL THC or 150ng/mL COC). The concentration of a drug is expressed in nanograms (ng) per milliliters (mL) or ng/mL. One nanogram is equal to one billionth of a gram and 1mL is equal to about 1/30 of a fluid ounce.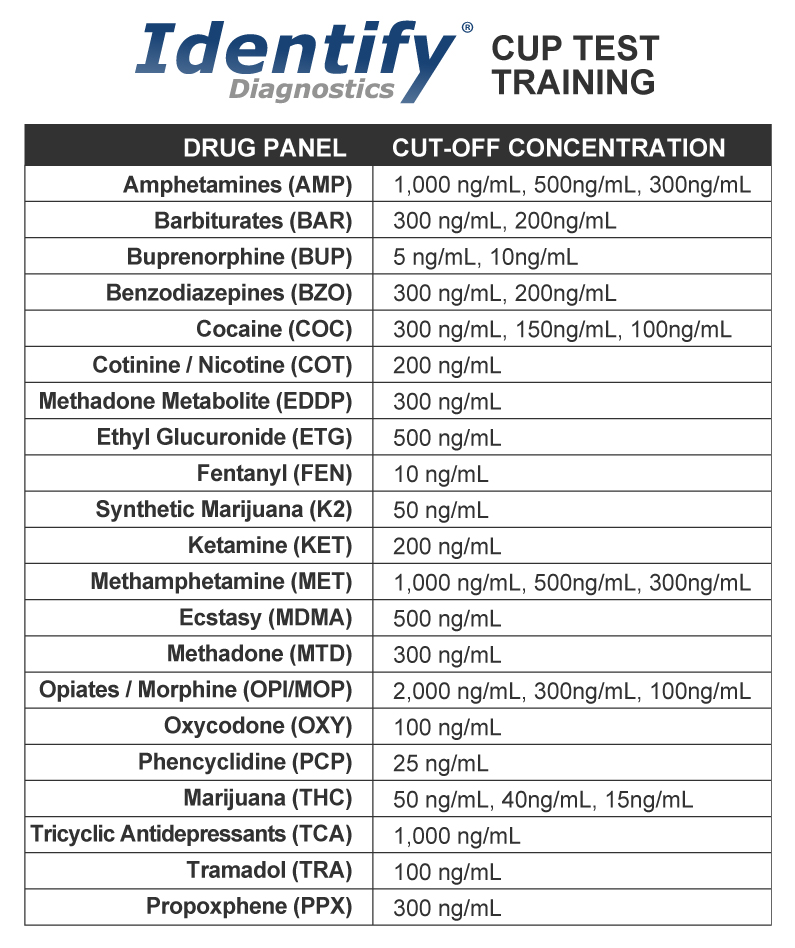
Storage And Materials
Identify Diagnostics Cup device should be stored at ambient room temperature (15-30 degrees° C/ 59-86 degrees° F). Verify expiration date is valid- Do NOT use beyond expiration date. Expiration date is stamped on each pouch and each box: MM/YYYY
Product Features And Benefits:
* Test up to 18 drugs simultaneously
* 18 Individual chambers- eliminate drug interpretation confusion
* Available with adulterant test (SVT)
* Unbreakable cup surface
* 510(k) Cleared strips available
* CLIA-Waived strips available
* Built in temperature strip on each cup
* Wide opening
* Easily match up arrows on lid and cup to lock firmly in place to prevent leaking.
* No tilting or tipping to activate
* Results in 5 minutes
* Results stable for 10 minutes
* Exclusive panel configurations to include EtG, fentanyl, K2/Spice and buprenorphine.
Procedure & Specimen Collection
1. Have available for use a test results form/record template
2. Require donor to present photo ID
3. Ask donor to remove any unnecessary outer clothing and empty all pockets
4. Keep all backpacks and purses out of restroom
5. Secure collection site:
6. Turn off water sources (Sink faucets)
7. Ensure blue toilet water
8. Remove all substances that could be used to adulterate specimen- (cleansers, disinfectants, soaps, etc)
9. Secure toilet tank or blue tank water
10. Instruct donor to wash hands in your sight BEFORE donating a sample in order to remove any residue that may interfere with the specimen collection.
11. Visibly inspect foil package containing cup to ensure package has not been compromised. Tear open foil pouch and remove test cup.
12. Issue device to donor. Have individual urinate directly into the Identify Diagnostics Test Cup. Instruct donor to fill cup above the minimum level of 30mL. This allows the temperature strip to activate and ensures enough specimen is available if additional confirmation laboratory testing is required.
13. Place lid on cup and turn until locked into place. There is an indicating line that should match the line on the lid to ensure lid is secured (check the cup and look for yourself).
14. Check temperature to ensure normal range from 90-100 Degrees Fahrenheit. If temperature does not fall within normal range, specimen is invalid.
Results Interpretation
Interpret negative test results as soon as the Control Line and Test Line appear. This may be as early as 30 seconds. Light lines on any drug test strip, IS A NEGATIVE. When a Control Line is present and any presence of a test line readable, no matter the intensity, is to be reported as a NEGATIVE.
Line intensity will vary from lot to lot and from specimen to specimen. DO NOT COMPARE THE LINE INTENSITIES TO OTHER TEST LINES ON THE DEVICE OR TO THE CONTROL LINE. Any presence of a test line at 5 minutes, no matter if it is less dark than others or is a broken line, is a NEGATIVE result for that drug strip.
Interpret a POSITIVE result when the Control Line is present and NO Test Line is present at 5 minutes. It is important to wait the full 5 minutes before test results are interpreted as Positive. Due to the chemical composition and chemistry of certain drugs, they may take longer than other strips for the test lines to form (up to 5 minutes).
Results are stable for 10 minutes, results read after 10 minutes are invalid. The testing cup may include a Specimen Validity Test Strip for the detection of specimen integrity which may include creatinine, nitrite, glutaraldehyde, pH, specific gravity, and oxidants. Please refer to the color chart included in your box of testing cups as colors may vary from lot to lot. The adulterant (specimen validity) pads must be read at the test read time listed in the color chart as the colors may change. Pad colors that change to a color NOT found listed in the normal range are to be read as abnormal.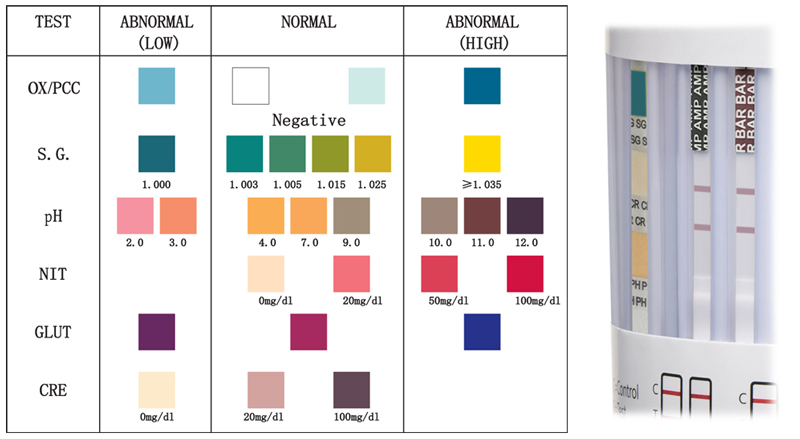
An Example of a Cup With a 3 Pad Adulterant Strip:
Compare the “SG’ strip for specific gravity to the color chart above and notice that the “SG” strip is blue, which is the color found under the Abnormal (low) column of the chart. Therefore, this specimen result is “Abnormal.” If the color of the pad is not found on the chart under the column “abnormal” it defaults to a normal reading. Please note that color varies on computer screens.
Line Intensities May Vary
Notice in the photo some of the bottom test lines are less bold than the control lines on top. These results are all negative, no matter how faint.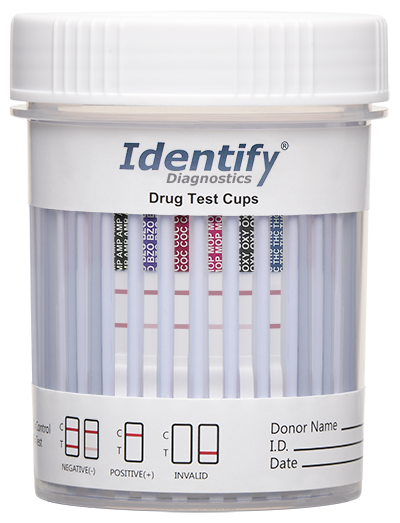
Key For Reading Drug Test Results
• Control Line (top line) = test valid
• No Control Line = test invalid (repeat)
• Test Line= test negative
• No Test Line = test positive
Invalid Results
The figure below is an example of an invalid test for COC and MOP. The other drug strips shown are negative. Due to the one drug per strip technology, each drug strip is to be read individually.
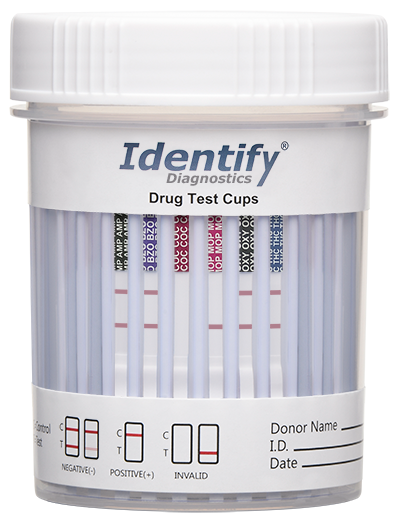
The following is an example of a positive test result for COC. 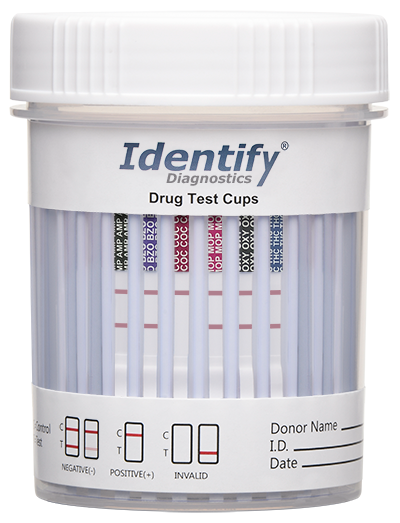
To view or download a copy of the Identify Diagnostics Drug Test Cup Quiz please see the icon below. ![]()
Please print out and complete your drug test cup training course quiz. Email the completed test to (Brad @ IdentifyDiagnostics.com) along with the name of the test taker. We will grade the quiz and email your personalized drug test cup training certificate once a perfect score has been achieved. If you have questions feel free to call us at 727-744-2967. Thank you.
