Identify Diagnostics USA
6 Panel Drug Test Cup - Identify Diagnostics USA CLIA Waived
- SKU:
- ID-US6-AZ25
- MPN:
- ID-US6
- Availability:
- Ships same day if by 3pm EST. Signature required delivery.
Description
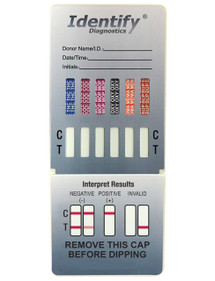
Identify Diagnostics USA - 6 Panel point of care drug test cup with - POCT
Availability:
Ships same day if by 3pm EST. Signature required delivery.
- MADE IN USA
- CLIA Waived
- FDA 510(K) Cleared
- Over The Counter OTC Cleared
- Urine temperature strip included
- Purchase in bulk or small quantities to meet your specific volume needs
- Choose from cup pack sizes of: 25, 50, 75, 100, 125, 150, 175, 200, 400, 600, 800 cups
- Join our flexible auto-ship program to help make reordering easy
- The expiration date is typically 11 months or longer of available shelf life
- For large orders please email a.lugo@medicaldistributiongroup.com or call (727)744-2967
Drugs Tested:
OXY - Oxycodone 100 ng/ml
BZO - Benzodiazepines 300 ng/ml
AMP - Amphetamines 500 ng/ml
OPI300 - Morphine / Opiates 300 ng/ml
COC - Cocaine 150 ng/ml
THC - Cannabinoid (Marijuana) 50 ng/ml
Our Made in the USA 6 panel drug test cup is ideal for pain management, family practice, Addiction medicine or primary care practice.
For the Identify Diagnostics USA drug test package CLIA insert please click the icon below:![]()
The 6 Panel Identify Diagnostics USA Drug Test Cup is a remarkable drug testing tool that is designed to help detect the presence of six commonly abused drugs in a person urine sample. This cup is proudly made in the USA and is CLIA waived and 510k cleared, which indicates that it meets the stringent regulatory standards of the United States. The 6 Panel Identify Diagnostics USA Drug Test Cup is an excellent option for businesses, healthcare providers, and law enforcement agencies that require accurate and reliable instant drug testing results.
The 6 Panel Identify Diagnostics USA Drug Test Cup is a urine-based drug testing kit that detects the presence of six common drug metabolites, including OXY - Oxycodone 100 ng/ml, BZO - Benzodiazepines 300 ng/ml, AMP - Amphetamines 500 ng/ml, OPI300 - Morphine / Opiates 300 ng/ml, COC - Cocaine 150 ng/ml, THC - Cannabinoid (Marijuana) 50 ng/ml. The test kit consists of a plastic cup with a lid, which contains panel strips that are designed to detect the presence of these six drugs. The test is straightforward to use and provides results within 5 minutes.
One of the key advantages of the 6 Panel Identify Diagnostics USA Drug Test Cup is its CLIA waived and 510k clearance status. CLIA stands for Clinical Laboratory Improvement Amendments, which are federal regulations that establish quality standards for laboratory testing. CLIA waived status indicates that the test has been approved by the US Department of Health and Human Services (HHS) as a simple and reliable test that can be used by non-laboratory personnel. 510k clearance is a regulatory requirement for medical devices, which ensures that the device is safe and effective for its intended use.
Another significant advantage of the 6 Panel Identify Diagnostics USA Drug Test Cup is its accuracy and reliability. The test is designed to provide accurate and reliable results, even in the presence of common adulterants that are often used to mask drug use. The test is also highly sensitive, meaning that it can detect even low levels of drug metabolites in urine samples. This feature makes it an ideal tool for drug screening programs in workplaces and healthcare facilities.
The 6 Panel Identify Diagnostics USA Drug Test Cup is also very easy to use. The test kit comes with clear instructions on how to collect a urine sample, how to perform the test, and how to interpret the results. The cup is designed with a leak-proof lid, which prevents spillage and ensures that the test remains hygienic and safe. The cup also has a temperature strip, which helps to ensure that the urine sample is fresh and has not been adulterated.
In conclusion, the 6 Panel Identify Diagnostics USA Drug Test Cup is a reliable, accurate, and easy-to-use drug testing tool that is ideal for businesses, healthcare providers, and law enforcement agencies. Its CLIA waived and 510k clearance status ensures that it meets the highest standards of quality and safety, and its sensitivity and specificity make it an ideal tool for drug screening programs. The 6 Panel Identify Diagnostics USA Drug Test Cup is an excellent investment for any organization that needs to ensure a drug-free workplace or environment.